
The major challenge
of the energy transition
An essential component of electric vehicles, lithium-ion batteries are a key technology in the decarbonization of our transportation systems. To address the supply issues surrounding the so-called “critical” metals used in their manufacture, recycling the materials found in end-of-life batteries appears to be an ideal solution. This solution is being explored within the Batteries axis of the PEPR, through the ‘Lulabat’ project, which aims to improve recycling processes.
Chronicle by the members of the Lulabat project
The skyrocketing demand for lithium-ion batteries has created unprecedented demand for a number of materials (cobalt, lithium, graphite, etc.) considered “critical” by the European Union (EU). In a constrained geopolitical context, the EU has adopted new regulations that encourage the transition from a linear economy to a circular economy, as evidenced by the principle of extended producer responsibility. Recycling these objects is a societal necessity and a matter of sovereignty for access to materials and the development of carbon-free mobility.
Lithium battery recycling processes exist and have been industrialized in China for several years for the recycling of lithium-ion batteries from electric vehicles. Europe is seeking to become independent from China by producing its own lithium-ion batteries and recycling them. This will only be possible if battery production and recycling processes are efficient, have a low environmental impact, and are economically viable. It is therefore necessary to improve existing processes so that they meet these objectives. The search for new technological building blocks to integrate into these recycling processes is therefore essential. The Lulabat project aims to contribute to this by providing new knowledge that can be transformed into technological innovations.
Current inefficient processes
The first lithium-ion battery recycling processes were based on pyrometallurgical operations, which involved treating batteries at high temperatures (> 1000 °C) to recover certain metals, mainly cobalt, nickel, and copper, while aluminum and lithium were lost in the slag and graphite was converted into CO2. These processes consume a lot of energy, have a low capacity to recover metals in the form required for reuse in the production of new batteries, and result in a total loss of lithium.
In order to produce materials compatible with battery production, better manage impurities, and reduce energy and environmental impacts, pyrometallurgy must be replaced by recycling processes combining mechanical and hydrometallurgical treatment:
Mechanical processing of lithium-ion batteries produces a concentrate that can be treated by hydrometallurgy, recovering all materials.
Hydrometallurgical processes rely on the use of leaching agents (often acidic solutions) to dissolve the metals contained in used battery materials, followed by chemical extraction, purification, and separation operations to produce various reusable salts for manufacturing new materials for lithium-ion battery production (electrodes, electrolyte salts).
Reducing the operating cost of such processes involves reducing the number of unit operations and minimizing effluents, while reducing reagent and energy consumption. Other strategies are also possible, based more specifically on materials science to regenerate the functionality of electrode materials without resorting to hydrometallurgy.

An unprecedented growth
Lithium-ion batteries play a central role in energy storage, particularly from intermittent sources (sun, wind, etc.), but also in electric mobility, two major challenges of the energy transition.
By 2030, depending on the scenario, global demand for lithium-ion batteries is expected to reach 2-3 TWh worldwide, with 80-90% dedicated to electric vehicles (with nearly a third in Europe), representing an increase of 30% each year. Over the same period, the amount of waste is expected to increase tenfold, which will have an impact on the recycling industry, which will have to evolve accordingly.
Research in the Batteries axis
The first phase of the LULABAT research program focuses in particular on the development of original technological building blocks and the assessment of their integration into a hydrometallurgical process. These technological building blocks, illustrated in Figure 1, are:
- Attrition leaching
The first step involves dissolving (or leaching) the powder from used batteries. The material to be dissolved, known as black mass (BM), is prepared through physical pre-treatment steps (grinding, pyrolysis, anode separation, etc.). The technological option studied for leaching the metals contained in BM is attrition leaching. The advantage of this process is that it refreshes the surface condition of the particles by continuously removing surface deposits that could slow down or block their leaching, thanks to the suspension of a solid medium (typically millimeter-sized silica beads) in the leaching reactor. In this complex reactive environment, the study focuses on exploring the operating conditions necessary for the selective dissolution of certain elements in BM, in particular lithium.
- Electrodialysis
Electrodialysis, although rarely used in current hydrometallurgical processes, is a mature industrial operation that enables efficient ion separation with reduced reagent consumption compared to traditional extraction and separation methods. In this part of the LULABAT program, we are evaluating the potential of this technology for lithium-ion battery recycling. We are looking for the optimal conditions for integrating electrodialysis into the first stage of the process, i.e., after leaching. The goal is to use electrodialysis for the selective extraction of lithium in order to produce battery-grade lithium hydroxide. At the same time, we are considering the integration of other unit operations downstream of electrodialysis, such as precipitation, crystallization, and liquid-liquid extraction, to recover other metals present in batteries in the form of sulfate, while reducing reagent consumption and effluent production.
- Oxidative precipitation
This is a chemical approach that aims to extract metals of interest as a complement or substitute for current methods. Oxidative precipitation offers advantages in terms of simplicity of industrial implementation, but also in terms of its complementarity with solvent extraction to reduce the number of extraction stages. Separation is possible in the absence of chloride, and its capacity has been demonstrated in advanced oxidation processes. As part of the LULABAT program, R&D work aims to understand the reaction pathways and mechanisms involved depending on the treatment parameters, which determine the efficiency and selectivity of the separation. This work should make it possible to identify the optimal operating conditions for evaluating the integration of the technology into recycling processes and the recovery of critical metals.
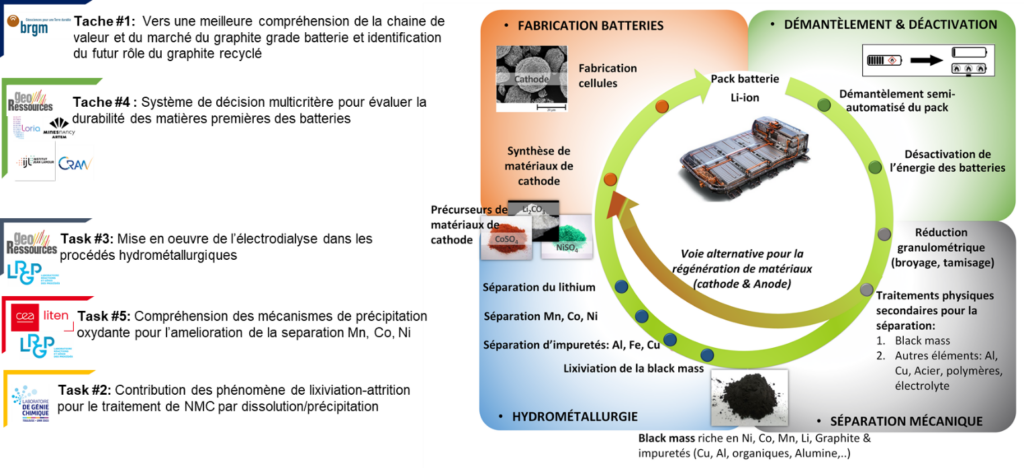
tasks mainly involve the hydrometallurgical process
More news


